
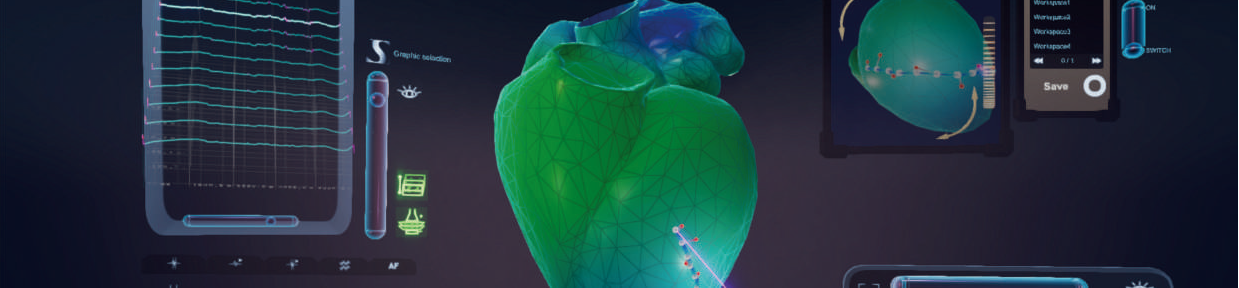
 VR-CARDIO
VR-CARDIO Market
The global market for wearable medical devices was valued at $35.6 billion in 2023. This market is estimated to grow from $45.0 billion in 2024 to $151.8 billion by the end of 2029, at a compound 2.1 mill annual growth rate (CAGR) of 27.5% from 2024 through 2029.
- Report code: HLC192D
- Publisher: BCC Publishing
- Date of publication: July 2024
- Category: Health maintenance
Clinical advantages of VR-CARDIO
-
Reduce hospital waiting list times
-
Cheaper than current systems
-
Minimize interventions due to false positives
-
Reduce operation time in the operating theatre
Timeline
- 2019 EIBT seal by ANCES (certifies that the company meets the criteria of innova- tion, technological development and growth potential).
- 2020 Innovative SME Seal of excellence awarded by the European Commission.
- 2021 Oracle Technology Startup Award EIC Accelerator (provides funding to support disruptive innovations).
- 2022 QIA Awards in potential innovation National. QIA Awards in potencial innovation International level. TRL6 VR-CARDIO: Prototype Demo Successfully Completed.
- 2023 First Health to Market Award. The EDF assigned Spika Tech to the Defense Tech projects SESIOP, REACT II and CASSATA. TRL7 VR-CARDIO: Real environment Demo Successfully completed.
- 2024 ISO 13485 Certification
for VR-CARDIO. TRL8 VR-CARDIO: Clinical
test validation. The EDF assigned Spika
Tech to the Defense Tech project EISNET. Recognition as an international success story of the
European Enterprise Network.
Around the world
-
 Singapore Australia Finland Sweden
Denmark
Singapore Australia Finland Sweden
Denmark
- NextRise Korea
- MWC Spain China USA
- ARAB HEALTH UAE
- HLTH EUROPE Germany
- COMMERCIAL MISSION Netherlands
- DMEA Canada
Team

-
 Carlos Zúñiga CEO
Carlos Zúñiga CEO -
 Esther Arnaiz President/ Founder
Esther Arnaiz President/ Founder
Medical Advisor
-
 José Ángel Cabrera MD, PhD
José Ángel Cabrera MD, PhD -
Xim Torrebella MD
Partners

